ME PAD Defibrillator (AED)
The ME PAD can be used at any place. The user gets all the necessary assistance as a voice instruction whereas voice volume is automatically adjusted to the ambient noise. Up to five operations of three hours of duration can be recorded on the internal SD memory card. At any time all the important data such as heart rhythm, energy output, time, etc. are available for the post-analysis.
In addition to the automatic power switch while using children pads, the output can be lowered manually. Thus children can be treated in emergency cases even if pediatric electrodes are not available. In daily, weekly and monthly intervals a test program is performed. All functions including battery performance and durability of the electrodes are automatically checked. An alarm will be given in case of errors.
The software of ME PAD is programmed according to the current guidelines of the ERC. The user is instructed through the entire resuscitation. The long-life Li-ion battery guarantees a standby time of up to five years or up to 200 shocks at full power.
| Automatic External Defibrillator (AED) ME PAD semi / ME PAD auto |
|
|
|
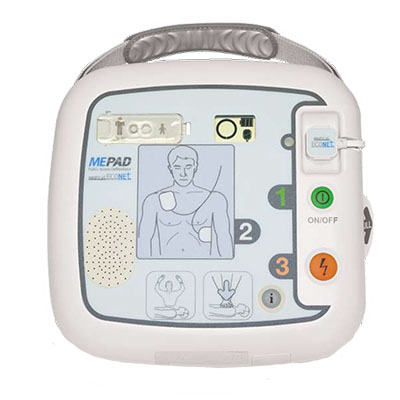 ME Pad |
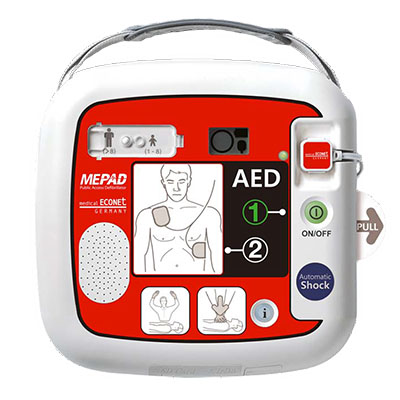 ME PAD Automatic |
Technical Data:
| ME PAD semi / ME PAD auto | |
| Defibrillation | |
| Output Energy | Adult - 150 Joule at 50 Ω Pediatric - 50 Joule at 50 Ω (common usage) |
| Charging Time | less than 8 seconds |
| Charging Time after CPR finished | at least 8 seconds |
| Waveform | E-cube two-phase (truncated expotential type) | ECG |
| Acquired ECG Lead | Lead II |
| Frequency Response | 1 Hz to 30 Hz |
| Impendance Range | 25 Ω bis 175 Ω (shock will not be delivered if the patients impedance is beyond this range) |
| Shockable Rhythms | Ventricular Fibrillation or Fast Ventricular Fibrillation |
| Sensitivity and Specificity | meets ANSI/AAMI DF80 guidelines | Operational Guidance |
| Control Devices | Power Button, i-Button, Shock Button (only ME PAD), Adult/ Pediatric Selection Switch |
| Status LCD | Displays device status, battery level and pads status |
| Speaker | Plays back voice instructions. The CU-SP1 analyzes the ambient noise level during a treatment operation. If ambient noise level is high, it automatically increases the voice instructions volume so that you can hear them clearly. | Self-Diagnostic Test |
| Auto | Power On Self-Test, Run-time Self-Test Daily, Weekly, and Monthly Self-Test |
| Manual | Battery Pack Insertion Test (done when the user inserts the battery pack into the battery pack compartment of the device) | Disposable Battery Pack |
| Battery Type | 12V DC, 4.2Ah LiMnO2, Disposable: Long-life |
| Capacity | At least 200 shocks for a new battery or 8 hours of operating time at room temperature |
| Standby Life (After inserting battery) | At least 5 years from the date of manufacture if stored and maintained in accordance with the instructions in this document. |
| Temperature Ranges | Operating: 0° - 43° C Storage: -20° - 60° C | Pads |
| Adult Defibrillation Pads | Electrode Area: 120 cm2 Cable Length: Total 120 cm (Inside the pouch: 95 cm, Outside the pouch: 25 cm) Shelf life: Up to 30 months from the date of manufacture |
| Pediatric Defibrillation Pads | Electrode Area: 46,43 cm2 Cable Length: Total 120 cm (Inside the pouch: 80 cm, Outside the pouch: 40 cm) Shelf life: Up to 24 months from the date of manufacture | Data Storage and Transfer |
| Internal Memory Data Capacity | 5 individual treatments, up to 3 hours per treatment |
| SD Card | External memory. Data may be copied from the internal memory to the SD Card |
| IrDA | For PC communications | Standards |
| Sealing | meets DIN EN 60529: IP55 |
| ESD | meets EN 61000-4-2:2001 |
| EMI (Radiated) | meets EN 60601-1-2 limits, method EN 55011:2007 + A2:2007, Group 1, Class B |
| EMI (Immunity) | meets IEC 60601-1-2 limits, method EN 61000-4-3:2006 +A1:2008 Level 3 (10V/m 80MHz to 2500MHz) |
| Vibration | Operating: Meets MIL-STD-810G Fig.514.6E-1, random Standby: Meets MIL-STD-810G Fig.514.6E-2, swept sine(helicopter) |
| Environmental Conditions | Operation: 0° - 43° C, 5% - 95% (non condensing) Standby: 0° - 43° C, 5% - 95% (non condensing) Transport: -20° - 60° C, 5% - 95% (non condensing), device only |
| Dimensions | 260 x 256 x 69,5 mm (WxLxH) |
| Weight | 2,4 kg including battery pack & pads |